ANTIBACTERIAL PEPTIDE SUBLANCIN ON THE GROWTH, ANTIOXIDANT CAPACITY, AND INTESTINAL HEALTH OF GRASS CARP (CTENOPHARYNGODON IDELLA)
-
摘要:
研究以草鱼(Ctenopharyngodon idella)为实验对象, 通过在基础饲料中添加0 (对照组, Control)、 0.9 g/kg (低剂量组, LD)、1.8 g/kg (中剂量组, MD)、2.7 g/kg (高剂量组, HD)的枯草芽孢杆菌源抗菌肽Sublancin, 采用酶联免疫吸附测定、组织切片HE染色、透射电镜扫描(TEM)及实时荧光定量PCR等技术探究Sublancin对草鱼生长、抗氧化能力及肠道健康的影响。结果发现: (1)与对照组相比, 中剂量组草鱼终末体重(FBW)、增重(WG)及增重率(WGR)显著提高, 中、低剂量组特定生长率(SGR)显著升高(P<0.05); (2)相较对照组, 抗菌肽组草鱼不同肠段的绒毛长度、宽度及肌层厚度显著增加, 不同肠段的隐窝深度显著降低(P<0.05); (3)与对照组相比, 中、低剂量组草鱼肠道微绒毛数量增加且排列更整齐, 紧密连接电子密度更高; (4)与对照组相比, 中、低剂量组草鱼血清总超氧化物歧化酶(T-SOD)活性显著升高(P<0.05), 中剂量组草鱼血清白细胞介素-22 (IL-22)水平显著升高, 抗菌肽组草鱼血清内毒素(LPS)含量显著降低, 抗菌肽组草鱼中肠claudin c、occludin及低剂量组草鱼中肠ZO-2紧密连接相关蛋白基因表达水平显著提高(P<0.05)。综上, 适量抗菌肽Sublancin能提高草鱼的生长性能、抗氧化能力, 增强肠道屏障, 对肠道健康有积极影响; 但过量抗菌肽会抑制草鱼生长, 减弱其氧化应激能力与肠道健康, 研究结果为抗菌肽在水产养殖业中的应用提供理论依据。
Abstract:Antimicrobial peptides (AMPs) are active substances with antimicrobial or bactericidal functions that can enhance the health of farmed animals. However, it is unknown whether AMPs can improve the intestinal health of grass carp (Ctenopharyngodon idella). In the present study, grass carp was fed with four different diets, including a control group without the antimicrobial peptide Sublancin, and three groups supplemented with Sublancin at doses of 0.9 g/kg (low dose, LD), 1.8 g/kg (middle dose, MD), and 2.7 g/kg (high dose, HD). We utilized a range of analytical methods, including enzyme-linked immunosorbent assay, hematoxylin and eosin tissue staining, transmission electron microscopy (TEM), and Real-time fluorescence quantitative PCR, to investigate the effects of the Sublancin on growth, antioxidant capacity, and intestinal health. The results showed that: (1) Compared to the control group, the final body weight (FBW), weight gain (WG), and weight gain rate (WGR) of grass carp in the MD group increased significantly. Additionally, the specific growth rate (SGR) elevated significantly in both the LD and MD groups (P<0.05). (2) The length and width of intestinal villi, as well as the thickness of the intestinal muscularis propria, exhibited a marked increase in grass carp treated with the antimicrobial peptide Sublancin, while crypts depth in different intestinal segments decreased significantly in the antimicrobial peptide group compared with the control group (P<0.05). (3) The number of intestinal microvilli exhibited a notable increase in both the LD and MD groups compared to the control group. Furthermore, the microvilli in the LD and HD groups were more tightly packed and had a higher density of securely connected electron-dense structures. (4) Compared with the control group, the serum total superoxide dismutase (T-SOD) activity increased significantly in both the LD and MD groups (P<0.05). Meanwhile, the content of serum interleukin-22 (IL-22) in the MD group increased significantly. Additionally, the antimicrobial peptide group significantly decreased the level of serum endotoxin (LPS) and increased the mRNA expression levels of midgut tight junction-related protein genes claudin c and occludin. There was a significant elevation in the mRNA expression level of ZO-2 in the LD group (P<0.05). In summary, the administration of appropriate amounts of the antimicrobial peptide Sublancin can improve the growth performance, enhance antioxidant capacity, strengthen intestinal barrier function, and promote overall intestinal health in grass carp. However, excessive antimicrobial peptides can inhibit the growth of grass carp and decrease oxidative stress capacity and intestinal health. These findings provide theoretical support for the application of antimicrobial peptides in aquaculture.
-
Keywords:
- Antibacterial peptide /
- Growth /
- Antioxidant capacity /
- Intestinal health /
- Ctenopharyngodon idella
-
水产养殖集约化发展的同时, 养殖密度不断提高、种质资源不断退化、病原体大量滋生、水环境污染加剧等一系列问题导致水产动物疾病频繁发生, 严重威胁水产养殖业的健康与可持续发展[1, 2]。肠炎是水产养殖中最常见的危害较严重的一类的疾病[3], 该病每年都会对我国水产养殖业造成相当大的损失[4]。病原感染、营养失衡、膳食微量元素缺乏、环境污染、动物遗传缺陷等均能导致鱼类肠炎的发生[5], 患病鱼常表现出相似的肠道症状: 肠道屏障损伤严重, 尤其是紧密连接结构破坏、细胞坏死、炎性细胞浸润和肠道通透性增加[5—8]。抗生素是治疗肠炎的常用药物, 但抗生素的使用往往会造成药物残留、耐药菌产生、肠道稳态受损、菌群平衡破坏及屏障功能障碍[9, 10]。寻找抗生素替代品, 预防肠炎的发生, 已成为水产养殖业亟需解决的问题[11]。
抗菌肽(Antimicrobial peptides, AMPs)是具有抗菌或杀菌活性的多肽类物质[12]。具有广谱抗菌活性的同时, AMPs也有独特的杀菌方式, 不会导致耐药菌的产生, 因而AMPs已经成为最具潜力的抗生素替代品[13]。此外, 畜禽中的研究发现, AMPs还能促进动物的生长[14], 调节动物肠道微生物区系, 增强动物免疫[15, 16], 提高肠道屏障功能, 促进机体健康[14, 17], 但目前抗菌肽在鱼类肠道健康中的作用还不甚清楚。
Sublancin是枯草芽孢杆菌分泌的具有2个二硫键和37个氨基酸的阳离子活性肽, 在pH 1.5—9.5功能稳定, 且能耐受极端的高温环境, 对革兰氏阳性菌具有显著的抗菌效果[18]。畜禽中的研究发现, Sublancin 能提高肉鸡(Gallus gallus)的生长性能[18], 缓解肉鸡的坏死性肠炎[19], 显示出较高的应用价值, 但Sublancin在鱼类中的相关研究仍较有限。
草鱼(Ctenopharyngodon idella)是全球最重要的养殖鱼类, 2022年我国草鱼养殖产量达590.48万吨, 占全国淡水鱼养殖总产量的21.78%, 在淡水养殖主要鱼类产量中排名第一[20, 21]。但草鱼养殖中肠炎发病率较高, 对草鱼养殖业造成较大的危害[3]。本实验以草鱼为研究对象, 通过在饲料中添加不同含量的抗菌肽Sublancin, 开展养殖实验, 探究Sublancin对草鱼生长、抗氧化及肠道屏障功能的影响, 为其在水产养殖业中的应用提供参考。同时, 研究结果将能够减少抗生素及药物的滥用, 推动我国水产养殖业绿色、健康及可持续发展。
1. 材料与方法
1.1 饲料准备
本研究使用的抗菌肽Sublancin由中农颖泰林州生物科园有限公司提供, 于常温避光的条件下保存。基础饲料配方及基本组成见表 1, 饲料经原料粉碎、过筛、充分混匀、膨化制成, 之后向饲料中添加不同剂量的抗菌肽Sublancin, 将喷洒不同浓度抗菌肽的饲料晾干后放置于4℃冰箱保存直至使用。养殖实验中不同组别饲料的使用情况如下: (1)对照(Control)组: 基础饲料; (2)低剂量(Low Dose, LD)组: 添加0.9 g/kg抗菌肽Sublancin的饲料; (3)中剂量(Middle Dose, MD)组: 添加1.8 g/kg抗菌肽Sublancin的饲料; (4)高剂量(High Dose, HD)组: 添加2.7 g/kg抗菌肽Sublancin的饲料。
表 1 饲料配方及基本组成Table 1. Feed formulation and basic composition原料成分Ingredient 含量Content (%) 对照Control 低剂量LD 中剂量MD 高剂量HD 豆粕Soybean meal 10 10 10 10 菜粕Rapeseed meal 14.62 14.62 14.62 14.62 菜饼Oilseed cake 12 12 12 12 鱼粉Fishmeal 18 18 18 18 玉米胚芽粕Corn germ meal 8 8 8 8 面粉Wheat flour 18 18 18 18 大豆油Soybean oil 3.5 3.5 3.5 3.5 L-赖氨酸盐酸盐L-Lysine hydrochloride 0.3 0.3 0.3 0.3 DL-蛋氨酸DL-methionine 0.2 0.2 0.2 0.2 乙氧基喹啉Ethoxyquinoline 0.03 0.03 0.03 0.03 氯化胆碱Choline chloride 0.3 0.3 0.3 0.3 磷酸二氢钙Ca(H2PO4)2·H2O 2.5 2.5 2.5 2.5 矿物质预混料Mineral premix1 0.3 0.3 0.3 0.3 维生素预混料Vitamin premix2 0.25 0.25 0.25 0.25 膨润土Bentonite 12 12 12 12 抗菌肽Sublancin (g/kg) 0 0.9 1.8 2.7 共计Total 100 100 100 100 营养水平Nutritional value 粗蛋白Crude protein 31.67 31.67 31.67 31.67 粗脂肪Crude lipid 6.83 6.83 6.83 6.83 蛋氨酸Met 0.81 0.81 0.81 0.81 赖氨酸Lys 2.09 2.09 2.09 2.09 注: 1矿物质预混料(g/kg): 碘酸钙, 5 g; 五水合硫酸铜, 24 g; 一水合硫酸亚铁, 150 g; 一水合硫酸锌, 74 g; 一水合硫酸锰, 13.5 g; 一水合硫酸镁, 350 g; 亚硒酸钠, 1.5 g; 氯化钴, 4 g; 沸石粉, 270 g; 稻壳粉, 108 g。 2维生素预混料(g/kg): 维生素B1, 8.2 g; 维生素B2, 12.8 g; 维生素B6, 9.3 g; 维生素B12, 1 g; 维生素D, 32.9 g; 维生素E, 248 g; 维生素K, 328 g; 烟酰胺, 35 g; 泛酸钙, 40 g; VC磷酸脂, 260 g; 肌醇, 53 g; 叶酸, 1.4 g; 生物素, 8.6 g; 乙氧基喹啉, 1.5 g; 稻壳粉, 279.8 g; 沸石粉, 0.5 gNote: 1 Mineral premix (g/kg): Calcium iodine, 5 g; CuSO4·5H2O, 24 g; FeSO4·H2O, 150 g; ZnSO4·H2O, 74 g; MnSO4·H2O, 13.5 g; MgSO4·H2O, 350 g; Na2SeO3, 1.5g; CoCl2, 4 g; Zeolite powder, 270 g; Rice husk powder, 108 g. 2 Vitamin premix (g/kg): Thiamin, 8.2 g; Riboflavin, 12.8 g; Pyridoxine, 9.3 g; Cyanocobalamine, 1 g; Vitamin D, 32.9 g; Vitamin E, 248 g; Vitamin K, 328 g; Niacinamide, 35 g; Calcium patotheniate, 40 g; VC phosphate, 260 g; Inositol, 53 g; Folic acid, 1.4 g; Biotin, 8.6 g; Ethoxyquin, 1.5 g; Rice husk powder, 279.8 g; Zeolite powder, 0.5 g 1.2 饲养实验
草鱼幼鱼购自湖北省洪湖市草鱼养殖场, 实验前将鱼苗运输至圆形养殖缸(500 L/缸)中暂驯化2月。之后选取规格一致, 体质健康的幼鱼252尾, 随机分入12个养殖桶(200 L/桶)中, 每桶21尾, 再将12桶鱼(平均体重46.85 g, 平均体长14.06 cm)随机分为4个组: 对照组、低剂量组、中剂量组及高剂量组, 每组3个重复。养殖实验开始后, 每日两次(9:00和17:00)投喂幼鱼至饱食, 20:00更换1/3的曝气水, 同时用吸污装置清理缸底排泄物, 持续投喂60d。实验期间随时注意观察鱼体健康情况, 保持水质状况良好, 水温为(28.5±0.5)℃, 光照周期12/12 (光照12h/黑暗12h), pH为7.5±0.2, 总氨氮含量低于0.2 mg/L, 亚硝酸盐含量低于0.01 mg/L。
1.3 样品采集
在养殖实验结束后, 使用浓度为100 mg/L的三卡因甲硫酸盐(MS-222)对草鱼进行麻醉。每桶随机取7尾鱼, 称量体重, 测定体长, 尾静脉采血, 采集的血液于4℃静置隔夜, 取上清置于–80℃冰箱保存, 用于后续血清生化指标的分析。将草鱼解剖, 分离内脏团、肝脏, 分别称重计量, 使用无菌镊子分离草鱼肠道, 准确区分前、中、后肠, 于各肠段同一位置取约1 cm肠道组织, 置入4%多聚甲醛固定液中保存, 进行 HE染色分析。同时采集肠段置于2.5%戊二醛固定液, 用于透射电子显微镜(TEM)下肠道超微结构的观察, 其余肠道组织经液氮冷冻后迅速存放于–80℃冰箱。本研究所有动物处理程序和实验均由中国科学院水生生物研究所伦理委员会审查批准。
1.4 生长性能分析
在养殖实验结束后, 从每个重复中随机取7尾鱼, 准确记录草鱼的终末体重、终末体长、内脏团重量及肝脏重量等生长相关数据, 按照下列公式准确计算肥满度(Condition fator, CF)、增重(Weight gain, WG)、特定生长率(Specific growth rate, SGR)、脏体比(Viscerosomatic index, VSI)及肝体比(Hepatosomatic index, HIS)等生长性能指标。
肥满度(CF, g/cm3)=100×体重/体长3
增重(WG, g)=终末体重–初始体重
增重率(WGR,%)=100×(终末体重–初始体重)/初体重
特定生长率(SGR, %/d)=100×(ln终末体重–ln初始体重)/天数
饵料系数(FCR)=摄食量/(终末体重–初始体重)
脏体比(VSI)=100×内脏团重/体重
肝体比(HIS)=100×肝脏重/体重
1.5 血清生化指标测定
根据南京建成生物工程研究所和江苏酶免实业有限公司生产的科研试剂盒说明书, 对采集的血清样本进行一系列特定生化指标的测定, 分析的指标包括: 总超氧化物歧化酶(T-SOD, U/mL)、丙二醛(MDA, nmol/mL)、内毒素(LPS, ng/L)及白细胞介素-22 (IL-22, ng/L)。
1.6 肠道组织HE染色
将采集的长度约1 cm的草鱼前、中、后肠分别放置于4%多聚甲醛中固定48h, 将组织经过(洗涤、脱水、透明、浸蜡、包埋)处理, 再将包埋的石蜡块进行切片, 于温水中展平, 利用载玻片捞起, 完成后续HE染色等步骤。使用正置荧光显微镜进行切片的拍照观察, 每张切片随机挑选5个部位, 使用Lightools软件对肠道绒毛长度(Villus height)、绒毛宽度(Villus width)、肌层厚度(Muscle thickness)与隐窝深度(Crypt depth)进行测量, 将计算的平均值作为本次实验的测量数据。
1.7 透射电子显微镜(TEM)扫描
将草鱼后肠组织置于2.5%戊二醛中进行固定, 以保持细胞的完整结构。再将样品放入磷酸盐缓冲液稀释的1%四氧化锇酸溶液中, 经过固定、脱水、包埋等步骤得到乙酸双氧铀和柠檬酸铅双重染色的切片, 以便在透射电子显微镜下观察。透射电镜切片的制作及观察由中国科学院水生生物研究所分析测试中心协助完成。
1.8 实时荧光定量检测
依据Takara的TRIzol Reagent说明书提取草鱼中肠组织的总RNA, 利用NanoDrop 2000超微量分光光度计对提取的RNA进行浓度和纯度的测定, 将经检测质量合格的RNA按照TaKaRa反转录试剂盒说明书逆转录合成cDNA, 利用TB Green Master Mix、CFX96TM实时PCR检测系统对cDNA完成实时荧光定量PCR(RT-qPCR)的分析, 通过2−∆∆Ct方法计算目标基因相对表达量, 实验中所用特异性引物序列见表 2, 选择β-actin作为内参基因, 进行标准化。
表 2 用于RT-qPCR的引物Table 2. Primers used for RT-qPCR目标基因Target gene 引物Primer sequence (5′—3′) 参考文献Reference 过氧化氢酶CAT GAAGTTCTACACCGATGAGG [22] CCAGAAATCCCAAACCAT 闭锁小带蛋白-2 ZO-2 TACAGCGGGACTCTAAAATGG [22] TCACACGGTCGTTCTCAAAG 跨膜蛋白-c claudin c GAGGGAATCTGGATGAGC [22] CTGTTATGAAAGCGGCAC 闭合蛋白occludin TATCTGTATCACTACTGCGTCG [22] CATTCACCCAATCCTCCA 黏蛋白-2 Mucin2 GAGTTCCCAACCCAACACAT [23] AAAGGTCTACACAATCTGCCC 肌动蛋白β-actin AGCCATCCTTCTTGGGTATG [22] GGTGGGGCGATGATCTTGAT 1.9 数据统计与分析
本实验所有数据通过SPSS Statistics 27.0软件进行单因素方差分析(One-way ANOVA), 结果以平均值±标准误(mean±SE)的形式进行表示, 并采用Duncan’s多重范围检验比较不同处理组间的差异, 显著性差异的判断标准为P值小于0.05 (P<0.05)。
2. 结果
2.1 生长性能
生长性能计算发现, 抗菌肽对草鱼的生长有促进作用, 其中, 中剂量组的终末体重、增重、增重率均显著高于对照组(P<0.05), 中、低剂量组的特定生长率显著高于对照组(P<0.05; 表 3)。
表 3 不同水平Sublancin对草鱼生长性能的影响Table 3. Effects of different supplemental levels of Sublancin on growth performance of C. idella组别Group Control LD MD HD 初始体重
IBW (g)46.16±
1.2545.87±
1.3547.21±
1.4548.17±
1.44初始体长
IBL (cm)14.03±
0.1213.99±
0.1814.04±
0.1314.19±
0.16终末体重
FBW (g)125.31±
6.02b144.16±
6.38ab149.63±
8.00a144.45±
7.31ab终末体长
FBL (cm)18.77±
0.3519.46±
0.3519.69±
0.4819.54±
0.33增重
WG (g)79.15±
6.02b98.28±
6.38ab102.41±
7.96a96.28±
7.31ab增重率
WGR (%)171.50±
13.05b214.30±
14.03ab216.75±
16.58a199.92±
15.22ab特定生长率
SGR (%/d)1.63±
0.08b1.88±
0.07a1.88±
0.08a1.78±
0.09ab注: 结果用均值±标准误表示, 同行的不同字母代表组间存在显著差异(P<0.05), 相同字母或无字母代表差异不显著(P>0.05); 下同Note: Values are expressed as mean±SEM, different letters indicate significant differences (P<0.05), the same letter or with no letter indicate no significant differences (P>0.05). The same applies below 2.2 形体指标
随着饲料中抗菌肽浓度的升高, 与对照组相比, 抗菌肽组草鱼的肝体比、脏体比明显降低, 而肥满度明显升高, 但这些变化在统计上均不显著(P>0.05; 表 4)。
表 4 不同水平Sublancin对草鱼形体指标的影响Table 4. Effects of different levels of Sublancin on morphology indexes of C. idella组别Group Control LD MD HD 肝体比HSI 2.29±0.09 2.24±0.08 2.14±0.08 2.25±0.07 脏体比VSI 10.69±0.53 10.24±0.56 10.13±0.31 10.62±0.43 肥满度CF (g/cm3) 1.88±0.05 1.95±0.06 1.98±0.09 1.91±0.04 2.3 肠道形态
肠道HE染色 随着抗菌肽含量的上升, 草鱼肠道绒毛长度、绒毛宽度及肌层厚度增加后下降, 而隐窝深度呈现相反趋势(表 5)。抗菌肽组草鱼前肠的绒毛长度显著高于对照组(P<0.05), 其中, 低剂量组草鱼肠绒毛最长; 中、高剂量组草鱼后肠的绒毛长度显著高于对照组(P<0.05), 其中, 中剂量组草鱼后肠绒毛最长。低剂量组草鱼前肠、低剂量组草鱼后肠及中剂量组草鱼中肠的绒毛宽度最宽, 且显著宽于对照组(P<0.05)。中剂量组草鱼前、中、后肠的肌层厚度均最高, 且显著高于对照组(P<0.05)。此外, 与对照组相比, 抗菌肽组草鱼肠道隐窝深度均显著降低(P<0.05)。
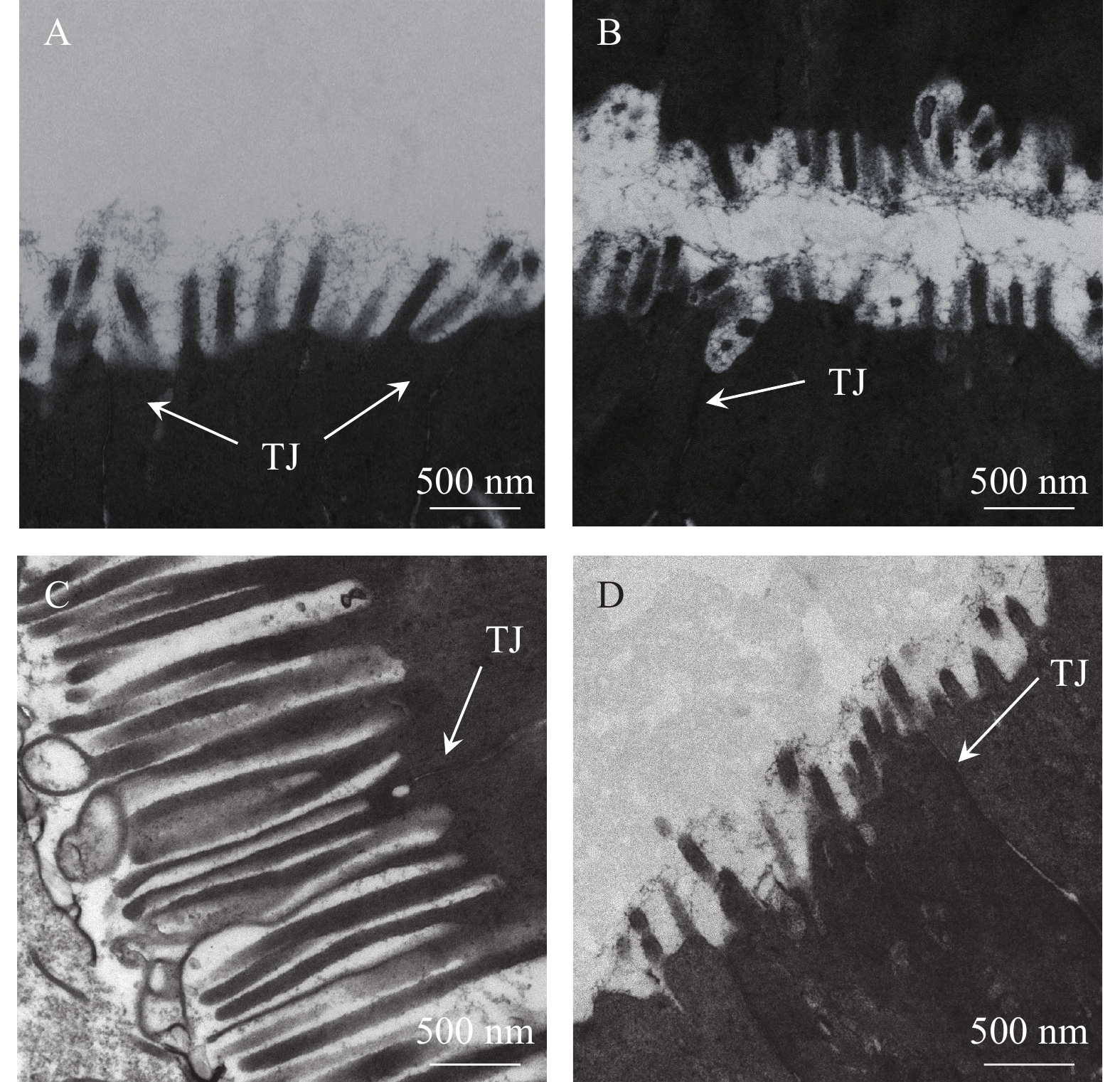
表 5 不同水平Sublancin对草鱼肠道形态的影响Table 5. Effects of different levels of Sublancin on the gut morphology of C. idella指标Item 部位 组别Group Control LD MD HD 绒毛长度Villus height (µm) 前肠 835.18±18.34b 1071.28±37.87a 1067.60±35.96a 1047.51±30.03a 中肠 655.51±21.21 699.44±31.35 673.50±15.42 647.22±19.86 后肠 519.45±26.94bc 571.37±18.30ab 614.39±22.70a 503.65±20.59c 绒毛宽度Villus width (µm) 前肠 130.77±3.92c 159.61±4.24a 145.19±4.12b 138.32±4.22bc 中肠 120.53±4.05b 135.92±3.46a 136.94±2.87a 129.35±3.25ab 后肠 109.61±3.32b 125.21±2.16a 122.67±2.21a 118.33±3.29a 肌层厚度Muscle thickness (µm) 前肠 143.45±4.52c 153.75±5.21bc 184.09±5.98a 159.31±4.41b 中肠 121.48±2.88c 134.75±2.32ab 140.54±2.26a 130.59±2.77b 后肠 128.37±3.61c 146.75±4.66ab 153.36 ±3.40a 136.85±4.89bc 隐窝深度Crypt depth (µm) 前肠 45.08±1.27a 41.34±1.54b 33.09±1.14c 36.39±1.08c 中肠 53.28±1.56a 42.40±1.14bc 40.34±0.88c 43.91±0.99b 后肠 56.00±1.57a 43.25±1.54c 47.35±1.35bc 49.89±1.58b 透射电子显微镜(TEM)观察 透射电镜结果显示, 与对照组相比, 中、低剂量组草鱼后肠微绒毛数量有所增加, 但高剂量组草鱼后肠微绒毛分布则较稀疏(图 1)。此外, 中剂量组草鱼后肠微绒毛变长, 排列更加整齐。肠上皮细胞紧密连接结构, 即紧密连接(Tight junctions, TJ), 在电子显微镜下表现为黑色的致密电子带, 位于上皮细胞顶端并沿着微绒毛根部向基底层延伸。与对照组相比, 中、低剂量组草鱼肠道上皮紧密连接结构更为整齐贴合, 电子密度更高。
2.4 抗氧化相关酶活性及基因的表达
与对照组相比, 中、低剂量组草鱼血清中丙二醛(MDA)含量降低, 高剂量组MDA水平增加, 但差异不显著(P>0.05)。此外, 低剂量组草鱼血清MDA含量显著低于高剂量组(P<0.05; 图 2A)。中、低剂量组血清中总超氧化物歧化酶(T-SOD)活性显著高于对照组(P<0.05; 图 2B)。低剂量组草鱼中肠组织的过氧化氢酶(CAT)基因表达水平最高, 但与对照组相比, 差异不显著(P>0.05; 图 2C)。
![]() 图 2 不同水平Sublancin对草鱼抗氧化相关酶活性及基因表达的影响A. MDA含量; B. T-SOD酶活性; C. 氧化应激相关基因mRNA水平; 不同字母代表不同组间存在显著性差异(P<0.05); 下同Figure 2. Effects of different levels of Sublancin on the relative expression of antioxidant related enzyme activity and gene of C. idellaA. Cotent of MDA; B. T-SOD activity; C. The gene mRNA expression related to oxidative stress Different letters indicate significant differences (P<0.05); The same applies below
图 2 不同水平Sublancin对草鱼抗氧化相关酶活性及基因表达的影响A. MDA含量; B. T-SOD酶活性; C. 氧化应激相关基因mRNA水平; 不同字母代表不同组间存在显著性差异(P<0.05); 下同Figure 2. Effects of different levels of Sublancin on the relative expression of antioxidant related enzyme activity and gene of C. idellaA. Cotent of MDA; B. T-SOD activity; C. The gene mRNA expression related to oxidative stress Different letters indicate significant differences (P<0.05); The same applies below2.5 肠道屏障及上皮黏蛋白相关基因的表达
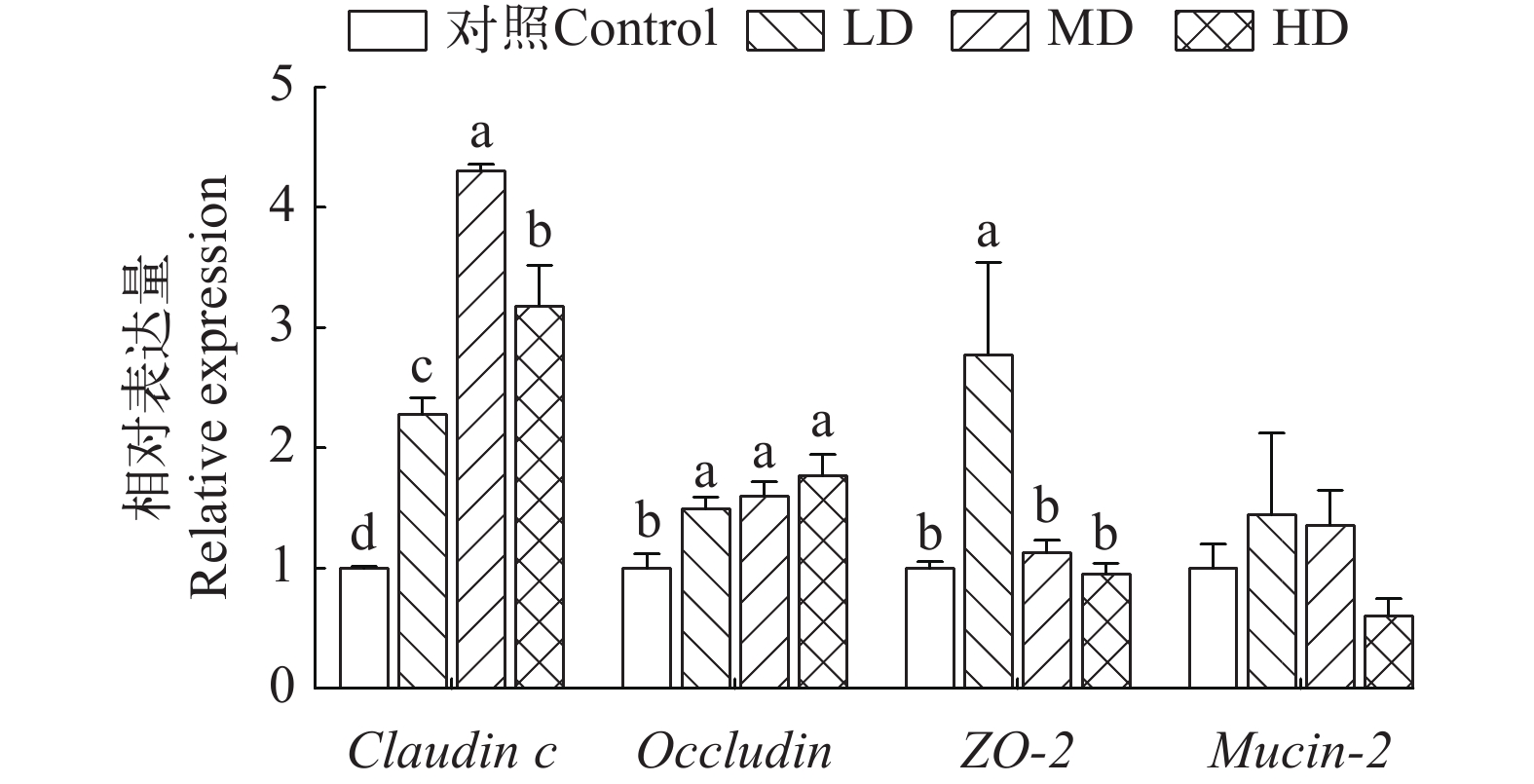
不同剂量的抗菌肽对草鱼中肠屏障功能相关基因的表达产生不同的调控效果(图 3)。中剂量组claudin c的mRNA表达水平显著提升, 之后下降, 且各组间存在显著性差异(P<0.05)。与对照组相比, 抗菌肽组occludin的相对表达水平显著上调(P<0.05), 且该上调作用随着抗菌肽剂量的提升而逐渐增强。ZO-2的mRNA表达水平在低剂量组中最高, 与其它组存在显著差异(P<0.05)。与对照组相比, 中、低剂量组草鱼中肠上皮细胞黏蛋白基因Mucin-2的表达水平明显上调, 然而在高剂量抗菌肽的作用下, Mucin-2的表达被抑制, 其mRNA表达水平明显下降(P>0.05)。
2.6 肠道通透性相关标志物分析
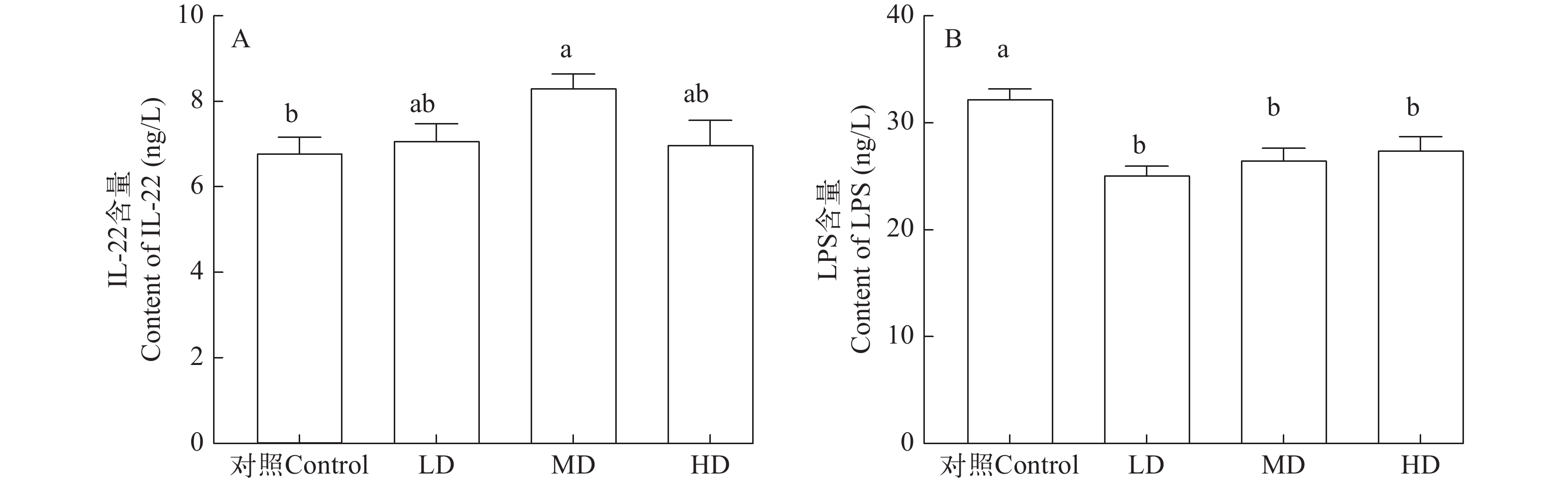
抗菌肽对草鱼肠道通透性影响显著(图 4)。与对照组相比, 不同剂量抗菌肽单独饲喂草鱼时, 血清中白细胞介素-22 (IL-22)含量升高; 特别是在中剂量抗菌肽处理下, IL-22的含量显著高于对照组(P<0.05), 且达到最高值(图 4A)。与对照组相比, 抗菌肽组草鱼血清中内毒素(LPS)含量均显著降低(P<0.05), 其中, 低剂量组LPS含量明显最低(图 4B)。
3. 讨论
3.1 抗菌肽对草鱼生长性能和形体指标的改善作用
前期的研究发现抗菌肽能够促进大口黑鲈(Micropterus salmoides)[24]、草鱼(Ctenopharyngodon idella)[25]与大黄鱼(Larimichthys crocea)[26]的终末体重、增重率及特定生长率等生长性能相关指标。本研究也有类似的发现, 饲喂适量的Sublancin对草鱼的生长性能有促进作用, 特别是在中剂量组的添加水平下效果最佳, 中剂量组草鱼的终末体重、增重、增重率和特定生长率相较对照组有显著提高。此外, 本研究还发现, 与中、低剂量组相比, 高剂量抗菌肽会抑制草鱼的生长, 这一结果与Chen等[27]和Hu等[23]的报道一致, 膳食补充过量抗菌肽将造成皱纹盘鲍(Haliotis discus hannai)特定生长率、草鱼终末体重及特定生长率的下降。以上结果表明养殖动物中抗菌肽Sublancin的添加需要适量。
肥满度、脏体比及肝体比是衡量形体指标的关键因子, 能够辅助揭示鱼体的健康状态及能量储存情况[28, 29]。本研究发现, 抗菌肽能明显降低草鱼的肝体比、脏体比, 且增加肥满度。这些结果与Ge等[26]和董晓庆等[30]的发现类似。在大黄鱼饲料中添加抗菌肽APSH-07, Ge等[26]发现鱼体肥满度提升, 脏体指数下降。董晓庆等[30]以天蚕素抗菌肽饲喂建鲤(Cyprinus carpio var. Jian), 发现鱼体的脏体指数、肝体指数分别下降。
3.2 抗菌肽对草鱼抗氧化能力的影响
血清生化参数能够反映动物的生理状况, 超氧化物歧化酶(SOD)、过氧化氢酶(CAT)及谷胱甘肽过氧化物酶(GPx)是动物体内常见的抗氧化相关的酶[31]。这些抗氧化指标能够反映鱼体对氧化应激的抵抗能力。膳食中补充抗菌肽能够提高大口黑鲈[24]与斜带石斑鱼(Epinephelus coioides)[32]血清中抗氧化酶(T-AOC、CAT、SOD)的水平, 降低脂质过氧化产物MDA含量。类似地, 在本研究中, 随着Subancin浓度的升高, 抗菌肽组草鱼血清总超氧化物歧化酶(T-SOD)水平和中肠CAT基因相对表达量先上升后下降, 尤其是中、低剂量组T-SOD水平显著高于对照组; 血清丙二醛(MDA)变化趋势则相反, 低剂量组草鱼血清中的丙二醛含量明显最低, 高剂量组丙二醛水平显著高于低剂量组。这些结果反映一定浓度范围内, 抗菌肽Sublancin能够通过增强草鱼血清抗氧化酶活性, 降低氧化应激造成的损伤, 进而对草鱼抗氧化防御产生积极作用, 特别是中剂量组效果最明显。但当超过某个阈值后, 过高浓度的抗菌肽Sublancin会减弱鱼体抵御氧化应激的能力。
3.3 抗菌肽对草鱼肠道形态的影响
肠道形态结构可被当作衡量动物消化吸收营养物质功能的指标[33]。特别是肠道绒毛长度、宽度及隐窝深度, 通常反映肠道消化吸收营养物质的有效面积与功能状态, 绒毛长度的提高、宽度的增加及隐窝深度的降低代表营养物质与肠道黏膜的接触面积增大, 从而增强机体消化吸收能力[24]。同时, 肌层厚度的增加意味着肠壁增厚, 进而提高肠道蠕动能力[34]。本研究发现, 一定剂量的抗菌肽能够显著改善草鱼肠道形态结构, 如: 增加肠道绒毛长度、绒毛宽度及肌层厚度, 降低隐窝深度, 说明抗菌肽Sublancin能够提高草鱼的消化吸收能力, 且本研究的抗菌肽Sublancin促进草鱼生长的部分结果也支持这一结论。本研究结果与 Liu等[35]和Dong等[36]的类似, 分别向草鱼、鲤(Cyprinus carpio)饲喂抗菌肽, 发现鱼体肠道绒毛长度提高, 隐窝深度降低, 促进鱼类的生长。
此外, 肠道超微结构透射电镜显示, 中、低剂量组微绒毛的数量有所增加, 但高剂量组微绒毛的分布变得更加稀疏。中、低剂量组草鱼肠道微绒毛长度增加, 排列更为整齐, 同时位于肠上皮细胞顶端, 沿着绒毛根部向下延伸的紧密连接得到增强, 即贴合更为紧密、更加规整、电子密度也更高。这说明一定剂量的抗菌肽Sublancin能够提高草鱼肠道机械屏障的完整性, 增强肠道屏障功能。类似地, Zhao等[17]发现抗菌肽MPX能有效缓解小鼠(Mus musculus)感染大肠杆菌后引起的空肠绒毛脱落、肠上皮细胞紧密连接的严重损伤, 显著改善肠道微绒毛的形态。
3.4 抗菌肽对草鱼屏障功能的调控作用
肠道机械屏障由肠上皮相邻细胞、固有膜、黏液层及胞间连接共同构成[37] 。肠上皮细胞上覆盖黏液层, 黏蛋白-2 (Mucin-2)是构成黏液层的关键部分, 其分泌量过低与肠道菌群功能失调、细菌易位、线粒体损伤有关[38, 39], 且紧密连接蛋白claudin c、occludin与ZO-2也是肠道机械屏障的重要组成部分[40]。机械屏障能够维持肠道内环境平衡、阻碍病原菌及毒素的入侵[41]。在本研究中, 草鱼肠道屏障功能相关基因(claudin c、occludin、ZO-2、Mucin-2)的表达水平在一定剂量抗菌肽的作用下有所上调, 特别是中、低剂量组的促进作用更显著。这说明一定浓度的抗菌肽能够增强草鱼肠道屏障的结构和功能, 有助于维持屏障完整性及稳定性, 进而保持肠道菌群平衡, 防止肠道病原微生物与毒素的入侵, 减少细菌易位, 降低鱼体患炎症性肠道疾病的风险。这与Fusco A等[42]和刘倚帆[43]的研究一致。Fusco A等[42]发现抗菌肽HBD-2、HBD-3在离体条件下, 通过诱导Mucin-2、Occludin等跨膜蛋白的表达, 增强屏障完整性, 保护宿主避免肠道通透性的改变。刘倚帆[43]将离体培养至亚融合状态的猪空肠上皮细胞经动物源抗菌肽处理后, 四种紧密连接蛋白Claudin、Occludin、ZO-1、ZO-2基因表达量显著增高, 组成肠道上皮屏障中至关重要的紧密连接形态完整性被有效保护。
分泌抗菌肽抵抗病原体的入侵, 形成紧密连接调节细胞旁通透性, 产生黏液维持肠道屏障是肠道上皮细胞的重要功能[44]。IL-22是肠道上皮执行细胞功能所需要的因子, 由3型先天淋巴细胞和T细胞产生[45, 46]。IL-22能够诱导潘氏细胞分泌AMP、刺激肠道上皮细胞的增殖、黏蛋白的合成以及促进黏膜液产生, 对维持肠道屏障完整性方面发挥重要作用[47, 48]。本研究发现中剂量抗菌肽处理下, 草鱼血清中IL-22的水平显著高于对照组, 这一结果表明, 适量的抗菌肽能够有效增强鱼体IL-22的分泌, 进而可能间接增强肠道屏障功能。
当肠道屏障功能受损时, 肠黏膜上皮细胞通透性升高, 易引发细菌易位的现象, 即细菌或其产物(内毒素)穿过肠黏膜屏障转移到肠外的其他部位, 例如, 血液或淋巴系统[49]。内毒素会破坏紧密连接蛋白, 对肠道稳态具有损伤作用[50]。血液中的内毒素含量能够反映黏膜屏障受损程度、细菌易位的水平[51]。在本研究中, 抗菌肽组草鱼血清LPS含量随Sublancin浓度的升高出现不同程度的下降, 说明抗菌肽减少细菌易位, 降低血清内毒素水平, 增强肠道上皮细胞的屏障功能。结合抗菌肽对肠道屏障功能的影响, 本研究推测抗菌肽Sulancin可能通过杀死肠内细菌或增强肠道上皮细胞屏障功能, 减少细菌移位, 降低草鱼血清的内毒素水平。
4. 结论
本研究表明, 在草鱼饲料中添加0.9、1.8 g/kg枯草芽孢杆菌源抗菌肽Sublancin可以增强生长性能和抗氧化能力、改善肠道形态结构、促进屏障功能相关基因的表达、降低血清内毒素含量以及提升IL-22细胞因子的含量, 进而维护草鱼肠道的稳态, 促进肠道健康。本研究为抗菌肽在水产养殖业中的应用提供依据和参考, 但抗菌肽促进草鱼生长、抗氧化能力及肠道健康的机制仍需要进一步探究。
-
图 2 不同水平Sublancin对草鱼抗氧化相关酶活性及基因表达的影响
A. MDA含量; B. T-SOD酶活性; C. 氧化应激相关基因mRNA水平; 不同字母代表不同组间存在显著性差异(P<0.05); 下同
Figure 2. Effects of different levels of Sublancin on the relative expression of antioxidant related enzyme activity and gene of C. idella
A. Cotent of MDA; B. T-SOD activity; C. The gene mRNA expression related to oxidative stress Different letters indicate significant differences (P<0.05); The same applies below
表 1 饲料配方及基本组成
Table 1 Feed formulation and basic composition
原料成分Ingredient 含量Content (%) 对照Control 低剂量LD 中剂量MD 高剂量HD 豆粕Soybean meal 10 10 10 10 菜粕Rapeseed meal 14.62 14.62 14.62 14.62 菜饼Oilseed cake 12 12 12 12 鱼粉Fishmeal 18 18 18 18 玉米胚芽粕Corn germ meal 8 8 8 8 面粉Wheat flour 18 18 18 18 大豆油Soybean oil 3.5 3.5 3.5 3.5 L-赖氨酸盐酸盐L-Lysine hydrochloride 0.3 0.3 0.3 0.3 DL-蛋氨酸DL-methionine 0.2 0.2 0.2 0.2 乙氧基喹啉Ethoxyquinoline 0.03 0.03 0.03 0.03 氯化胆碱Choline chloride 0.3 0.3 0.3 0.3 磷酸二氢钙Ca(H2PO4)2·H2O 2.5 2.5 2.5 2.5 矿物质预混料Mineral premix1 0.3 0.3 0.3 0.3 维生素预混料Vitamin premix2 0.25 0.25 0.25 0.25 膨润土Bentonite 12 12 12 12 抗菌肽Sublancin (g/kg) 0 0.9 1.8 2.7 共计Total 100 100 100 100 营养水平Nutritional value 粗蛋白Crude protein 31.67 31.67 31.67 31.67 粗脂肪Crude lipid 6.83 6.83 6.83 6.83 蛋氨酸Met 0.81 0.81 0.81 0.81 赖氨酸Lys 2.09 2.09 2.09 2.09 注: 1矿物质预混料(g/kg): 碘酸钙, 5 g; 五水合硫酸铜, 24 g; 一水合硫酸亚铁, 150 g; 一水合硫酸锌, 74 g; 一水合硫酸锰, 13.5 g; 一水合硫酸镁, 350 g; 亚硒酸钠, 1.5 g; 氯化钴, 4 g; 沸石粉, 270 g; 稻壳粉, 108 g。 2维生素预混料(g/kg): 维生素B1, 8.2 g; 维生素B2, 12.8 g; 维生素B6, 9.3 g; 维生素B12, 1 g; 维生素D, 32.9 g; 维生素E, 248 g; 维生素K, 328 g; 烟酰胺, 35 g; 泛酸钙, 40 g; VC磷酸脂, 260 g; 肌醇, 53 g; 叶酸, 1.4 g; 生物素, 8.6 g; 乙氧基喹啉, 1.5 g; 稻壳粉, 279.8 g; 沸石粉, 0.5 gNote: 1 Mineral premix (g/kg): Calcium iodine, 5 g; CuSO4·5H2O, 24 g; FeSO4·H2O, 150 g; ZnSO4·H2O, 74 g; MnSO4·H2O, 13.5 g; MgSO4·H2O, 350 g; Na2SeO3, 1.5g; CoCl2, 4 g; Zeolite powder, 270 g; Rice husk powder, 108 g. 2 Vitamin premix (g/kg): Thiamin, 8.2 g; Riboflavin, 12.8 g; Pyridoxine, 9.3 g; Cyanocobalamine, 1 g; Vitamin D, 32.9 g; Vitamin E, 248 g; Vitamin K, 328 g; Niacinamide, 35 g; Calcium patotheniate, 40 g; VC phosphate, 260 g; Inositol, 53 g; Folic acid, 1.4 g; Biotin, 8.6 g; Ethoxyquin, 1.5 g; Rice husk powder, 279.8 g; Zeolite powder, 0.5 g 表 2 用于RT-qPCR的引物
Table 2 Primers used for RT-qPCR
目标基因Target gene 引物Primer sequence (5′—3′) 参考文献Reference 过氧化氢酶CAT GAAGTTCTACACCGATGAGG [22] CCAGAAATCCCAAACCAT 闭锁小带蛋白-2 ZO-2 TACAGCGGGACTCTAAAATGG [22] TCACACGGTCGTTCTCAAAG 跨膜蛋白-c claudin c GAGGGAATCTGGATGAGC [22] CTGTTATGAAAGCGGCAC 闭合蛋白occludin TATCTGTATCACTACTGCGTCG [22] CATTCACCCAATCCTCCA 黏蛋白-2 Mucin2 GAGTTCCCAACCCAACACAT [23] AAAGGTCTACACAATCTGCCC 肌动蛋白β-actin AGCCATCCTTCTTGGGTATG [22] GGTGGGGCGATGATCTTGAT 表 3 不同水平Sublancin对草鱼生长性能的影响
Table 3 Effects of different supplemental levels of Sublancin on growth performance of C. idella
组别Group Control LD MD HD 初始体重
IBW (g)46.16±
1.2545.87±
1.3547.21±
1.4548.17±
1.44初始体长
IBL (cm)14.03±
0.1213.99±
0.1814.04±
0.1314.19±
0.16终末体重
FBW (g)125.31±
6.02b144.16±
6.38ab149.63±
8.00a144.45±
7.31ab终末体长
FBL (cm)18.77±
0.3519.46±
0.3519.69±
0.4819.54±
0.33增重
WG (g)79.15±
6.02b98.28±
6.38ab102.41±
7.96a96.28±
7.31ab增重率
WGR (%)171.50±
13.05b214.30±
14.03ab216.75±
16.58a199.92±
15.22ab特定生长率
SGR (%/d)1.63±
0.08b1.88±
0.07a1.88±
0.08a1.78±
0.09ab注: 结果用均值±标准误表示, 同行的不同字母代表组间存在显著差异(P<0.05), 相同字母或无字母代表差异不显著(P>0.05); 下同Note: Values are expressed as mean±SEM, different letters indicate significant differences (P<0.05), the same letter or with no letter indicate no significant differences (P>0.05). The same applies below 表 4 不同水平Sublancin对草鱼形体指标的影响
Table 4 Effects of different levels of Sublancin on morphology indexes of C. idella
组别Group Control LD MD HD 肝体比HSI 2.29±0.09 2.24±0.08 2.14±0.08 2.25±0.07 脏体比VSI 10.69±0.53 10.24±0.56 10.13±0.31 10.62±0.43 肥满度CF (g/cm3) 1.88±0.05 1.95±0.06 1.98±0.09 1.91±0.04 表 5 不同水平Sublancin对草鱼肠道形态的影响
Table 5 Effects of different levels of Sublancin on the gut morphology of C. idella
指标Item 部位 组别Group Control LD MD HD 绒毛长度Villus height (µm) 前肠 835.18±18.34b 1071.28±37.87a 1067.60±35.96a 1047.51±30.03a 中肠 655.51±21.21 699.44±31.35 673.50±15.42 647.22±19.86 后肠 519.45±26.94bc 571.37±18.30ab 614.39±22.70a 503.65±20.59c 绒毛宽度Villus width (µm) 前肠 130.77±3.92c 159.61±4.24a 145.19±4.12b 138.32±4.22bc 中肠 120.53±4.05b 135.92±3.46a 136.94±2.87a 129.35±3.25ab 后肠 109.61±3.32b 125.21±2.16a 122.67±2.21a 118.33±3.29a 肌层厚度Muscle thickness (µm) 前肠 143.45±4.52c 153.75±5.21bc 184.09±5.98a 159.31±4.41b 中肠 121.48±2.88c 134.75±2.32ab 140.54±2.26a 130.59±2.77b 后肠 128.37±3.61c 146.75±4.66ab 153.36 ±3.40a 136.85±4.89bc 隐窝深度Crypt depth (µm) 前肠 45.08±1.27a 41.34±1.54b 33.09±1.14c 36.39±1.08c 中肠 53.28±1.56a 42.40±1.14bc 40.34±0.88c 43.91±0.99b 后肠 56.00±1.57a 43.25±1.54c 47.35±1.35bc 49.89±1.58b -
[1] Du Y, Hu X, Miao L, et al. Current status and development prospects of aquatic vaccines [J]. Frontiers in Immunology, 2022(13): 1040336.
[2] 昝子叶, 柯飞, 赵威山, 等. 芽孢表面展示系统及其在水产疫苗研发中的应用 [J]. 水生生物学报, 2024, 48(4): 694-706.] doi: 10.7541/2024.2023.0259 Zan Z Y, Ke F, Zhao W S, et al. Spore surface display system and its application in aquatic vaccine development [J]. Acta Hydrobiologica Sinica, 2024, 48(4): 694-706. [ doi: 10.7541/2024.2023.0259
[3] Wu N, Xu X, Wang B, et al. Anti-foodborne enteritis effect of galantamine potentially via acetylcholine anti-inflammatory pathway in fish [J]. Fish & Shellfish Immunology, 2020(97): 204-215.
[4] 农业农村部渔业渔政管理局, 全国水产技术推广总站. 2023中国水生动物卫生状况报告 [M]. 北京: 中国农业出版社, 2023: 2-3.] Fisheries and Fisheries Administration Bureau of the Ministry of Agriculture and Rural Affairs. National Fisheries Technology Extension Station, China Fisheries Association. 2023 Aquatic Animal Health in China [M]. Beijing: China Agriculture Press, 2023: 2-3. [
[5] Zhang H, Ran C, Teame T, et al. Research progress on gut health of farmers teleost fish: a viewpoint concerning the intestinal mucosal barrier and the impact of its damage [J]. Reviews in Fish Biology and Fisheries, 2020, 30(4): 569-586. doi: 10.1007/s11160-020-09614-y
[6] Ouyang P, Huang S, Wei W, et al. Cinnamaldehyde treats largemouth bass non-lethal bacterial enteritis by regulating gut morphology, barrier, inflammation, microbiota and serum biochemistry [J]. Aquaculture, 2024(581): 740463.
[7] 余桂娟, 杨沛, 戴济鸿, 等. 饲料中添加大豆皂甙对大菱鲆幼鱼生长和肠道健康的影响 [J]. 水产学报, 2019, 43(4): 1104-1115.] Yu G J, Yang P, Dai J H, et al. Effects of dietary soyasaponins on the growth performance and intestinal health of juvenile turbot(Scophthalmus maximus) [J]. Journal of Fisheries of China, 2019, 43(4): 1104-1115. [
[8] Liu Y, Chen Z, Dai J, et al. Sodium butyrate supplementation in high-soybean meal diets for turbot (Scophthalmus maximus L.): effects on inflammatory status, mucosal barriers and microbiota in the intestine [J]. Fish & Shellfish Immunology, 2019(88): 65-75.
[9] Bondad-Reantaso M G, MacKinnon B, Karunasagar I, et al. Review of alternatives to antibiotic use in aquaculture [J]. Reviews in Aquaculture, 2023, 15(4): 1421-1451. doi: 10.1111/raq.12786
[10] Miller R A, Harbottle H. Antimicrobial drug resistance in fish pathogens [J]. Microbiology Spectrum, 2018, 6(1): 501-520.
[11] Lv R, Sun N, Mao C, et al. Prevention and potential repair of colitis: beneficial effects and regulatory mechanisms of food-derived anti-inflammatory peptides [J]. Critical Reviews in Food Science and Nutrition, 2024, 64(23): 8184-8202. doi: 10.1080/10408398.2023.2197068
[12] 董晓庆, 姜丹, 曲桂娟, 等. 抗菌肽对鱼类肠道菌群及免疫功能影响的研究进展 [J]. 饲料工业, 2024, 45(2): 43-48.] Dong X Q, Jiang D, Qu G J, et al. Research progress of antimicrobial peptides in fish intestinal flora and immune function [J]. Feed Industry, 2024, 45(2): 43-48. [
[13] 吴家明, 李芹. 抗菌肽的分类、作用机制与应用研究 [J]. 安徽农学通报, 2024, 30(2): 101-107.] doi: 10.3969/j.issn.1007-7731.2024.02.021 Wu J M, Li Q. Classification, mechanism of action and application of antimicrobial peptides [J]. Anhui Agricultural Science Bulletin, 2024, 30(2): 101-107. [ doi: 10.3969/j.issn.1007-7731.2024.02.021
[14] 郑雪玥, 黄熠, 贺海桥, 等. 抗菌肽和中草药添加剂对肉鸡生长性能、血清生化指标和肠道形态的影响 [J]. 动物营养学报, 2023, 35(1): 230-239.] Zheng X Y, Huang Y, He H Q, et al. Effects of antimicrobial peptides and Chinese herbal medicine additives on growth performance, serum biochemical indices and intestinal morphology of broilers [J]. Chinese Journal of Animal Nutrition, 2023, 35(1): 230-239. [
[15] Zhang X, Zhao Q, Wen L, et al. The effect of the antimicrobial peptide plectasin on the growth performance, intestinal health, and immune function of yellow-feathered chickens [J]. Frontiers in Veterinary Science, 2021(8): 688611.
[16] Daneshmand A, Kermanshahi H, Sekhavati M H, et al. Effects of cLFchimera peptide on intestinal morphology, integrity, microbiota, and immune cells in broiler chickens challenged with necrotic enteritis [J]. Scientific Reports, 2020, 10(1): 17704. doi: 10.1038/s41598-020-74754-x
[17] Zhao X Q, Wang L, Zhu C L, et al. Oral administration of the antimicrobial peptide mastoparan X alleviates enterohemorrhagic Escherichia coli-induced intestinal inflammation and regulates the gut microbiota [J]. Probiotics and Antimicrobial Proteins, 2024, 16(1): 138-151. doi: 10.1007/s12602-022-10013-x
[18] 丁修良, 赵建飞, 王帅, 等. 抗菌肽Sublancin对肉鸡生长性能、养分利用及盲肠菌群的影响 [J]. 动物营养学报, 2018, 30(7): 2690-2699.] doi: 10.3969/j.issn.1006-267x.2018.07.029 Ding X L, Zhao J F, Wang S, et al. Effects of antimicrobial peptide sublancin on growth performance, nutrient utilization and cecal microbiota of broilers [J]. Chinese Journal of Animal Nutrition, 2018, 30(7): 2690-2699. [ doi: 10.3969/j.issn.1006-267x.2018.07.029
[19] Wang S, Zeng X F, Wang Q W, et al. The antimicrobial peptide sublancin ameliorates necrotic enteritis induced by Clostridium perfringens in broilers [J]. Journal of Animal Science, 2015, 93(10): 4750-4760. doi: 10.2527/jas.2015-9284
[20] 农业农村部渔业渔政管理局, 全国水产技术推广总站, 中国水产学会. 2023中国渔业统计年鉴 [M]. 北京: 中国农业出版社, 2023: 25.] Bureau of Fisheries and Fishery Administration, Ministry of Agriculture and Rural Affairs, National General Station of Aquatic Technology Promotion, Chinese Fisheries Society. China fishery statistical yearbook 2023 [M]. Beijing: China Agriculture Press, 2023: 25. [
[21] 杨婉愉, 巫丽云, 张月星, 等. 饲料添加谷氨酸对草鱼生长、抗氧化能力和肌肉品质性状的影响 [J]. 水生生物学报, 2024, 48(9): 1509-1518.] Yang W Y, Wu L Y, Zhang Y X, et al. Dietary supplementation with glutamate on growth, antioxidant capacity, and muscle quality of grass carp(Ctenopharyngodon idella) [J]. Acta Hydrobiologica Sinica, 2024, 48(9): 1509-1518. [
[22] Qin L, Xiang J, Xiong F, et al. Effects of Bacillus licheniformis on the growth, antioxidant capacity, intestinal barrier and disease resistance of grass carp (Ctenopharyngodon idella) [J]. Fish & Shellfish Immunology, 2020(97): 344-350.
[23] Hu Q Y, Wu P, Feng L, et al. Antimicrobial peptide Isalo scorpion cytotoxic peptide (IsCT) enhanced growth performance and improved intestinal immune function associated with Janus kinases (JAKs)/signal transducers and activators of transcription (STATs) signalling pathways in on-growing grass carp (Ctenopharyngodon idella) [J]. Aquaculture, 2021(539): 736585.
[24] Li S, Chi S, Cheng X, et al. Effects of antimicrobial peptides on the growth performance, antioxidant and intestinal function in juvenile largemouth bass, Micropterus salmoides [J]. Aquaculture Reports, 2020(16): 100252.
[25] Huo X, Wang P, Zhao F, et al. High-efficiency expression of a novel antimicrobial peptide I20 with superior bactericidal ability and biocompatibility in Pichia pastoris and its efficiency enhancement to aquaculture [J]. Aquaculture, 2024(579): 740149.
[26] Ge H, Wang Q, Chen H, et al. Effects of antimicrobial peptide APSH-07 on the growth performance, anti-oxidation responses, stress resistance and intestine microbiota in large yellow croaker Larimichthys crocea [J]. Aquaculture Nutrition, 2020, 26(3): 715-726. doi: 10.1111/anu.13031
[27] Chen F, Li X, Wu Y, et al. Influences of dietary antimicrobial peptide APSH-07 on the growth performance, immune response and vibriosis resistance of abalone Haliotis discus hannai Ino [J]. Aquaculture Nutrition, 2020, 26(5): 1736-1747. doi: 10.1111/anu.13124
[28] Seppänen E, Kuukka H, Voutilainen A, et al. Metabolic depression and spleen and liver enlargement in juvenile Arctic charr Salvelinus alpinus exposed to chronic parasite infection [J]. Journal of Fish Biology, 2009, 74(3): 553-561. doi: 10.1111/j.1095-8649.2008.02144.x
[29] Abdel-Razek N. Antimicrobial activities of chitosan nanoparticles against pathogenic microorganisms in Nile Tilapia, Oreochromis niloticus [J]. Aquaculture International, 2019, 27(5): 1315-1330. doi: 10.1007/s10499-019-00388-0
[30] 董晓庆, 张东鸣, 陈玉珂, 等. 抗菌肽对建鲤生长性能和脏体指数及肌肉成分的影响 [J]. 中国畜牧杂志, 2015, 51(15): 47-51.] doi: 10.3969/j.issn.0258-7033.2015.15.011 Dong X Q, Zhang D M, Chen Y K, et al. Effects of antibacterial peptide on growth performances, viscera index and muscle composition in common carp(Cyprinus carpio) [J]. Chinese Journal of Animal Science, 2015, 51(15): 47-51. [ doi: 10.3969/j.issn.0258-7033.2015.15.011
[31] Wang Y, Wu Y, Wang Y, et al. Antioxidant properties of probiotic bacteria [J]. Nutrients, 2017, 9(5): 521. doi: 10.3390/nu9050521
[32] Su Y L, Chen G, Chen L S, et al. Effects of antimicrobial peptides on serum biochemical parameters, antioxidant activity and non-specific immune responses in Epinephelus coioides [J]. Fish & Shellfish Immunology, 2019(86): 1081-1087.
[33] Awad W A, Ghareeb K, Abdel-Raheem S, et al. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens [J]. Poultry Science, 2009, 88(1): 49-56. doi: 10.3382/ps.2008-00244
[34] Cao K, Wang Y, Li M, et al. Supplementation of a multienzyme complex, an organic acid-essential oil complex, and prebiotic alone or in combination affects growth, nutrient utilization, and immune function of rainbow trout (Oncorhynchus mykiss) [J]. Aquaculture Nutrition, 2022(1): 1068537.
[35] 刘淑林. 复合抗菌肽对草鱼生长、抗氧化及免疫的影响[D]. 广州: 华南农业大学, 2020: 15.] Liu S L. Effects of compound antimicrobial peptides feed on growth performance, antioxidant activity, and immunity of grass carp (Ctenopharyngodon idella)[D]. Guangzhou: South China Agricultural University, 2020: 15.
[36] Dong X Q, Qu G J, Chen Y K, et al. Effects of antimicrobial peptides on growth, morphology of foregut villi and related genes mRNA expression in the common carp (Cyprinus carpio) [J]. Aquaculture Research, 2019, 50(7): 1752-1761. doi: 10.1111/are.14041
[37] 陈秀梅, 王桂芹, 单晓枫, 等. 鱼类肠道屏障损伤与肠道炎症发生发展关系的研究进展 [J]. 河南农业科学, 2022, 51(5): 1-9.] Chen X M, Wang G Q, Shan X F, et al. Research progress on the relationship between intestinal barrier damage and intestinal inflammation development in fish [J]. Journal of Henan Agricultural Sciences, 2022, 51(5): 1-9. [
[38] Huang Z, Wu H, Fan J, et al. Colonic mucin-2 attenuates acute necrotizing pancreatitis in rats by modulating intestinal homeostasis [J]. FASEB Journal, 2023, 37(7): e22994. doi: 10.1096/fj.202201998R
[39] Borisova M A, Achasova K M, Morozova K N, et al. Mucin-2 knockout is a model of intercellular junction defects, mitochondrial damage and ATP depletion in the intestinal epithelium [J]. Scientific Reports, 2020, 10(1): 21135. doi: 10.1038/s41598-020-78141-4
[40] Chang X, Kang M, Shen Y, et al. Bacillus coagulans SCC-19 maintains intestinal health in cadmium-exposed common carp (Cyprinus carpio L. ) by strengthening the gut barriers, relieving oxidative stress and modulating the intestinal microflora [J]. Ecotoxicology and Environmental Safety, 2021(228): 112977.
[41] Zhang C, Zhao X H, Yang L, et al. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, microflora, and barrier integrity in broilers [J]. Poultry Science, 2017, 96(12): 4325-4332. doi: 10.3382/ps/pex266
[42] Fusco A, Savio V, Donniacuo M, et al. Antimicrobial peptides human beta-defensin-2 and-3 protect the gut during Candida albicans infections enhancing the intestinal barrier integrity: in vitro study [J]. Frontiers in Cellular and Infection Microbiology, 2021(11): 666900.
[43] 刘倚帆. 动物源抗菌肽的分子改良及其对猪肠道上皮屏障功能的保护作用研究 [D]. 杭州: 浙江大学, 2012: 78-81.] Liu Y F. Molecular design of animal antimicrobial peptides and study their protective effects on intestine epithelial barrier function [D]. Hangzhou: Zhejiang University, 2012: 78-81. [
[44] Iftekhar A, Sigal M. Defence and adaptation mechanisms of the intestinal epithelium upon infection [J]. International Journal of Medical Microbiology, 2021, 311(3): 151486. doi: 10.1016/j.ijmm.2021.151486
[45] Parks O B, Pociask D A, Hodzic Z, et al. Interleukin-22signaling in the regulation of intestinal health and disease [J]. Frontiers in Cell and Developmental Biology, 2015(3): 85.
[46] Yue R, Wei X, Hao L, et al. Promoting intestinal antimicrobial defense and microbiome symbiosis contributes to IL-22-mediated protection against alcoholic hepatitis in mice [J]. Frontiers in Immunology, 2023(14): 1289356.
[47] Patnaude L, Mayo M, Mario R, et al. Mechanisms and regulation of IL-22-mediated intestinal epithelial homeostasis and repair [J]. Life Sciences, 2021(271): 119195.
[48] Klotskova H B, Kidess E, Nadal A L, et al. The role of interleukin-22 in mammalian intestinal homeostasis: friend and foe [J]. Immunity, Inflammation and Disease, 2024, 12(2): e1144. doi: 10.1002/iid3.1144
[49] Wang Y H. Current progress of research on intestinal bacterial translocation [J]. Microbial Pathogenesis, 2021(152): 104652.
[50] Ghareeb K, Awad W A, Böhm J, et al. Impact of luminal and systemic endotoxin exposure on gut function, immune response and performance of chickens [J]. World’s Poultry Science Journal, 2016, 72(2): 367-380. doi: 10.1017/S0043933916000180
[51] 葛耀植, 黄红兰. 肠黏膜屏障功能障碍与细菌易位研究进展 [J]. 医学综述, 2018, 24(6): 1072-1076.] doi: 10.3969/j.issn.1006-2084.2018.06.007 Ge Y Z, Huang H L. Research advances of intestinal barrier dysfunction and bacterial translocation [J]. Medical Recapitulate, 2018, 24(6): 1072-1076. [ doi: 10.3969/j.issn.1006-2084.2018.06.007



 下载:
下载: